why ketones do not give fehling test Fehling’s test: objective, principle, reagents, procedure and result
Chemistry is a fascinating field of study that deals with the structure, properties, and reactions of materials. The reactions of aldehydes, ketones, and phenols are one of the most intriguing areas of study in this field. Recently, I came across some interesting data on this topic.
Reactions of Aldehydes, Ketones, and Phenols
 Aldehydes, ketones, and phenols are organic compounds that contain carbonyl groups. The carbonyl group is a functional group that consists of a carbon atom double-bonded to an oxygen atom. Aldehydes have a carbonyl group at the end of a carbon chain, whereas ketones have a carbonyl group in the middle of a carbon chain. Phenols have a hydroxyl group (-OH) attached to a benzene ring.
Aldehydes, ketones, and phenols are organic compounds that contain carbonyl groups. The carbonyl group is a functional group that consists of a carbon atom double-bonded to an oxygen atom. Aldehydes have a carbonyl group at the end of a carbon chain, whereas ketones have a carbonyl group in the middle of a carbon chain. Phenols have a hydroxyl group (-OH) attached to a benzene ring.
The reactions of aldehydes, ketones, and phenols are similar in many respects. One of the most important reactions is the nucleophilic addition reaction, in which a nucleophile (an electron-rich species) attacks the carbonyl group and adds to it. For example, aldehydes and ketones react with hydrogen cyanide (HCN) to form cyanohydrins:
 Phenols, on the other hand, undergo nucleophilic substitution reactions in which a nucleophile replaces the -OH group. For example, phenol reacts with bromine water (Br2/H2O) to form 2,4,6-tribromophenol:
Phenols, on the other hand, undergo nucleophilic substitution reactions in which a nucleophile replaces the -OH group. For example, phenol reacts with bromine water (Br2/H2O) to form 2,4,6-tribromophenol:
 Fehling’s Test
Fehling’s Test
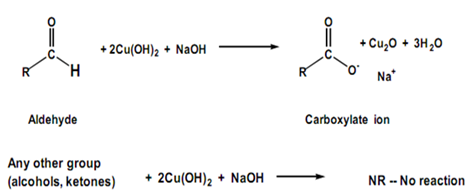
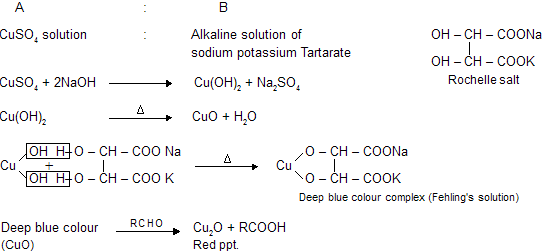
Fehling’s test is a common test used to distinguish between aldehydes and ketones. The test is based on the ability of aldehydes to reduce Cu2+ ions to Cu+ ions, which results in the formation of a red precipitate of Cu2O. Ketones, on the other hand, do not react with Fehling’s reagent because they cannot be oxidized by Cu2+ ions. Therefore, no red precipitate is formed in the case of ketones.
The Fehling’s reagent is a mixture of two solutions: Fehling’s A (aqueous CuSO4) and Fehling’s B (aqueous NaOH and sodium potassium tartrate). Equal volumes of Fehling’s A and B are mixed and heated to boiling, and then the test sample is added. If an aldehyde is present, a red precipitate of Cu2O is formed. If a ketone is present, no red precipitate is formed.
In conclusion, the reactions of aldehydes, ketones, and phenols are fascinating and complex. These reactions are crucial in many industrial processes, as well as in everyday life. Understanding the properties and reactivity of these compounds is critical for the development of new technologies and applications in various fields of chemistry.
If you are searching about One Part of Chemistry: Reactions of Aldehydes, Ketones And Phenols you’ve visit to the right page. We have 5 Images about One Part of Chemistry: Reactions of Aldehydes, Ketones And Phenols like One Part of Chemistry: Reactions of Aldehydes, Ketones And Phenols, Fehling’s Solution: Definition, Example, and Mechanism and also [Solved] Why do α-hydroxy ketones give Tollens’ test? | 9to5Science. Read more:
One Part Of Chemistry: Reactions Of Aldehydes, Ketones And Phenols
 1chemistry.blogspot.co.ukketones aldehydes test reactions fehling reagent brady solution chemistry part formation result
1chemistry.blogspot.co.ukketones aldehydes test reactions fehling reagent brady solution chemistry part formation result
Fehling’s Test: Objective, Principle, Reagents, Procedure And Result
 www.onlinebiologynotes.comtest solution fehling formula principle fehlings copper reagents oxide reaction reagent glucose lactose aldehyde aldehydes sugar reducing fructose positive solutions
www.onlinebiologynotes.comtest solution fehling formula principle fehlings copper reagents oxide reaction reagent glucose lactose aldehyde aldehydes sugar reducing fructose positive solutions
[Solved] Why Do α-hydroxy Ketones Give Tollens’ Test? | 9to5Science
![[Solved] Why do α-hydroxy ketones give Tollens’ test? | 9to5Science](https://i.stack.imgur.com/uXW80.png) 9to5science.comWhat Are Ketones? - Perfect Keto Exogenous Ketones
9to5science.comWhat Are Ketones? - Perfect Keto Exogenous Ketones
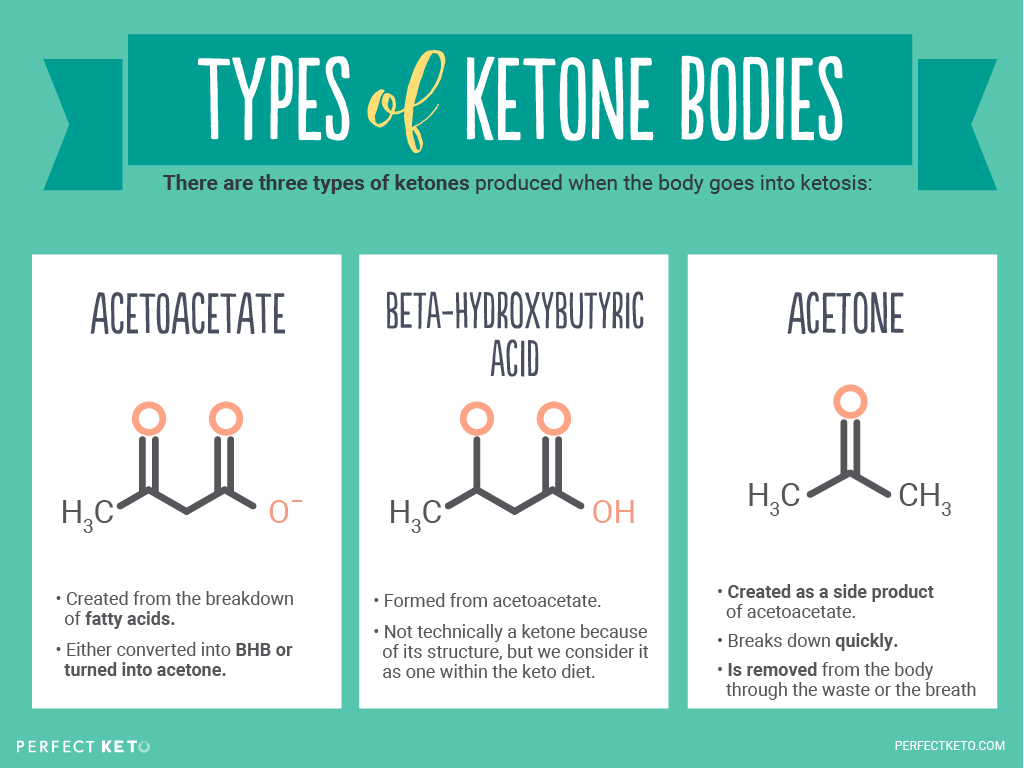 www.perfectketo.comketone ketones acetone bodies acetoacetate body bhb types why diet keto ketogenic levels fat beta hydroxybutyrate ketosis acid look does
www.perfectketo.comketone ketones acetone bodies acetoacetate body bhb types why diet keto ketogenic levels fat beta hydroxybutyrate ketosis acid look does
Fehling’s Solution: Definition, Example, And Mechanism
 www.chemistrylearner.comfehling fehlings mechanism chemistrylearner
www.chemistrylearner.comfehling fehlings mechanism chemistrylearner
One part of chemistry: reactions of aldehydes, ketones and phenols. Ketones aldehydes test reactions fehling reagent brady solution chemistry part formation result. Ketone ketones acetone bodies acetoacetate body bhb types why diet keto ketogenic levels fat beta hydroxybutyrate ketosis acid look does